Do age-associated B-cells contribute to the pathogenesis of rheumatoid arthritis?
Do age-associated B-cells contribute to the pathogenesis of rheumatoid arthritis?
Lead supervisor - Professor John Isaacs, Newcastle University
Additional supervisors - Dr Amy Anderson, Newcastle University; Professor Andrew Mellor, Newcastle University; Dr Dagmar Scheel-Toellner, University of Birmingham and Dr Arthur Pratt, Newcastle University
Aims
To understand the role of age-associated B-cells (ABC) in rheumatoid arthritis (RA) pathogenesis. More specifically to
- Enumerate and phenotype (transcriptomic and proteomic profile) ABC in relevant tissue and/or peripheral blood from early and established RA, as well as age-matched disease controls (other inflammatory and non-inflammatory arthritides) and healthy controls
- Investigate the function of ABC in terms of
• Migratory ability
• Autoantibody production
• Cytokine production
• Antigen processing and presentation
• Effect on T-cell differentiation - Investigate whether ABC correlate with response to rituximab in RA patients
Project overview
Rheumatoid arthritis (RA) is a complex heterogeneous disease (1). T-cells play a central role in disease pathogenesis (2). However, the presence of autoantibodies, such as anti-citrullinated peptide antibodies, years before the clinical development of disease (3), and the efficacy of rituximab, a B-cell depleting therapy (4), highlight a pathogenic role for B-cells. A novel subset of B-cells termed age-associated B-cells (ABC) have recently been described in autoimmune disease (5). These cells are CD19highCD21-CD11c+ and are increased in the peripheral blood (PB) of elderly women with autoimmune disease. A similar B-cell population in mice develops via a TLR7-dependent mechanism and these cells are refractory to B-cell receptor and CD40 stimulation but do respond to TLR7 and TLR9 stimulation resulting in immunoglobulin and cytokine production (5-6). In addition, murine ABC efficiently present antigen and favour Th17 polarisation (6). A CD21-/low B-cell population, which express autoreactive antibodies and are anergic, has been reported to be increased in RA PB (7). ABC may be a blood precursor of a novel subset of pro-inflammatory B-cells found in inflamed synovium which express FcRL4 and RANK-L (8).
Our work investigating ABC in RA has demonstrated increased proportions of these cells in RA compared to other inflammatory diseases (Figure 1).
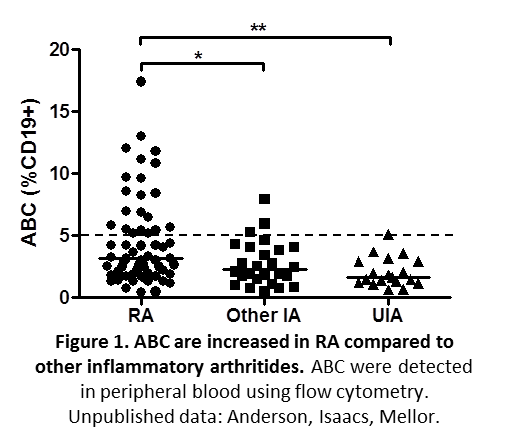
In addition there is a link between autoantibody production and the proportion of these cells in RA patients (Figure 2).
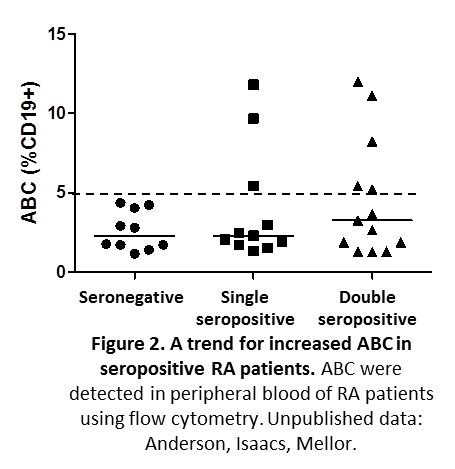
Interestingly, ABC express high levels of endogenous indoleamine 2,3 dioxygenase (IDO) (Figure 3) and may resemble a subset of murine IDO-expressing dendritic cells found in lymphoid tissues of mice with malignancies, chronic infections and SLE-like autoimmune syndromes (9-11).
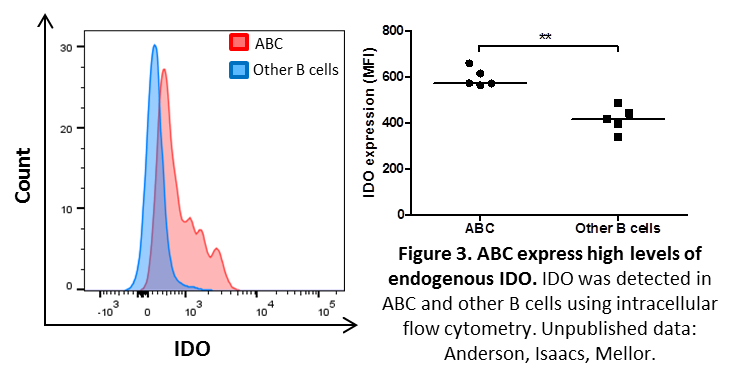
Hypotheses
- ABC represent a terminally differentiated, activated and pathogenic B-cell population in RA, and are increased in a subset of patients.
- ABC home to sites of inflammation and perpetuate inflammation and disease pathogenesis via the secretion of autoantibodies and skewing of T-cell responses to a Th17 phenotype.
- The proportion of ABC in RA patients will correlate with response to rituximab treatment.
In this project we will investigate the link between ABC and RA pathogenesis by carrying out detailed characterisation of the presence, phenotype and pathogenic role of ABC in the synovium and PB of patients with early and established RA, as well as correlating the proportions of ABC to rituximab treatment response. This project will lead to new insight into RA pathogenesis and the potential identification of a new cellular targets and stratification tools for the treatment of this condition.
Techniques
- Cell isolation from peripheral blood and tissues (magnetic bead selection and FACS)
- Primary cell culture
- Multi-parameter flow cytometry
- Extraction of RNA
- Nanostring for transcriptomic profiling
- ELISA/MSD immunoassay
- Antigen processing and presentation
- Migration assays
- T-cell proliferation assay
- T-cell differentiation assays
References
1. McInnes and Schett N Engl J Med 2011; 365:2205-19
2. Cope et al. p Rheumatol Clin Exp Rheumatol 2007; 25 (Suppl. 46): S4-S11
3. Deane Curr Rheumatol Rep 2014; 16:419
4. Edwards et al. N Engl J Med 2004; 350:2572-81
5. Rubtsov et al. Blood 2011; 118(5):1305-15
6. Hao et al. Blood 2011; 118(5):1294-304
7. Isnardi et al. Blood 2010; 115(24):5026–5036
8. Yeo et al. Ann Rheum Dis 2015; 74(5): 928–935
9. Munn et al. JCI 2004; 114(2):280-290
10. Johnson et al. PNAS 2010; 107(23):10644-10648
11. Huang et al. Int Rev Immunol 2010; 29:133-135




